Adreesh Ghoshal’s rather well researched article on why batteries catch fire on Medium is being reposted in full.
https://adreesh-ghoshal.medium.com/how-lithium-ion-batteries-in-evs-catch-fire-9d166c5b3af1
Although rare, Lithium-ion batteries do tend to catch fire. After investigations into a number of battery-related fires, some of the reasons have been identified. One of the most important, called thermal runaway, is when the entire battery-package overheats, resulting in a fire.
Why is this alarming? Well, that is because from tiny pacemakers to million-dollar cars and even spacecraft, we’re surrounded by billions of Li-ion-powered devices. Chances are pretty high that you’re reading this article on one.
So is your handheld device going to explode in your face? Not really. But you never know.
What we will attempt to, however, is to list everything we know about Li-ion fire hazards.

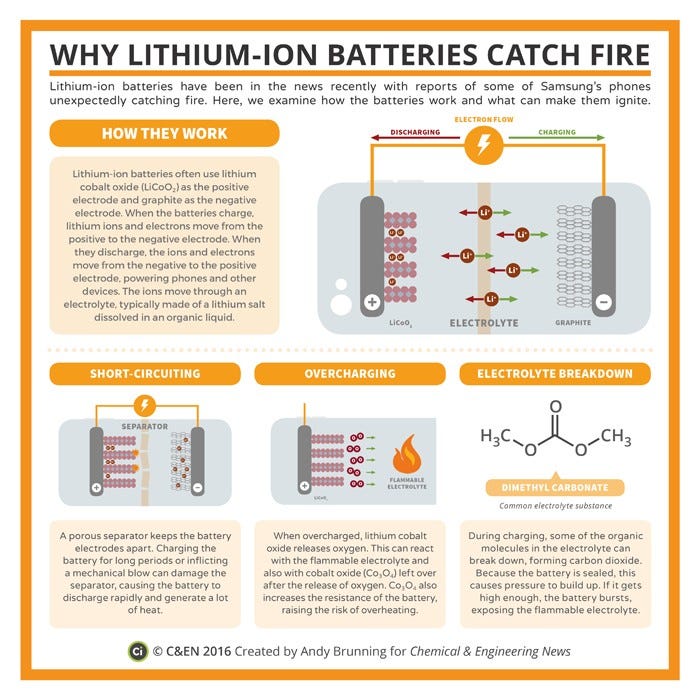
How They Work
Like all batteries, Li-ion batteries have a cathode, an anode, and an electrolyte. The cathode, typically made of a lithium-based metal, releases electrons. These electrons travel through an electrolyte, which is an organic compound consisting of lithium salts. The carbon-based anode accepts the electrons, setting up a flow of electric current. This discharges the battery
How They’re Built
A full battery pack consists of a number of cells, a voltage regulator, battery management systems and cooling systems. We’re, however, going to look at the cell, which is generally either shaped like a normal AAA cell or like a brick (that’s the one used in EVs). The cell consists of a cathode, an anode, a separator, and a vent hole. The separator is a thin plastic filter that only allows ions to pass through.
The Chemistry
Since this branch of chemistry only concerns electrons, it’s called electrochemistry. The cathode is made of lithium cobalt oxide. The anode is made out of graphite, and the electrolyte is made of a lithium-ion-based fluid with either ethylene carbonate or diethyl carbonate as the solvent.
How They’re Charged
While refueling a car, you’re filling an empty space with fluid (gas, for example). While charging a battery, however, you’re sending back an electric current from the anode to the cathode. The voltage of the current is higher than that produced by a cell (about 3.7 volts). This replenishes the electrons lost by the cathode.

Why They Heat Up
Every chemical reaction releases energy. When we eat food, calories are released. When we burn wood, we get a fire, another source of heat. So, during the charge-discharge cycle, too, heat is released. According to tests, the temperature inside a lithium-ion battery can reach up to 271 degrees. This heat is generated because the electrolyte and the anode offer electrical resistance to the reverse voltage created during charging. The one analogy we can think of is driving down the right (wrong) side of the road.
The Nio Fires
After posting a $460 million loss in June, Chinese EV maker Nio is in serious trouble. It crossed an overall $5 billion loss in value within 5 years of launch. They recently issued a huge recall over a potential battery hazard. And the problem, they say, is a short circuit, leading to an electrical fire. Out of the 17,500 vehicles they sold, 4,800 ES8 SUVs are on the list. This move came after multiple reports of cars self-igniting.

Short Circuit?
What is a short circuit? When an electric current “jumps”, it causes a short. In wiring, it’s most often due to cracked insulation. A short in a Li-ion-based battery is due to a damaged separator. Yes, that ultra-thin membrane we told you about? If that breaks, it causes massive over-voltage, causing a general heating up of things. And then cars catch fire.
Damaged Separators?
The separator is a thin plastic sheet. And by thin, we mean very, very, very thin. As thin as a strand of hair. Since it’s supposed to filter ions, it is an extremely delicate piece of tech. Since it’s so fragile, the separator breaks if the battery suffers an impact. If this happens, there is an uncontrolled reaction until the cathode gets discharged. Such a rapid discharge heats up the electrolyte, causing a fire.
High Energy Density?
A lithium-ion battery is four times as powerful as the “low tech” lead-acid battery. This was originally the reason why companies spend billions trying to eke out more power from Li-ion packs. But, because it can conduct a high-energy reaction, this releases a lot of heat and, even worse, in some cases, hydrogen. The result? Boom!
Thermal Runaway
Ah, the elephant in the room. Consider a stack of dominos. When you topple one, the rest collapse, one after another. Right? This is exactly what happens during thermal runaway. When the separators get ruptured, a lot of heat is released, and this causes the electrolyte to grow hot over time. Since high-density battery packs are the norm, hundreds of such cells are packed together to create one battery. Two or more such batteries are called a battery pack. If one cell overheats, the meltdown will spread and will continue until the entire pack is destroyed. This is a thermal runaway.
Over Charging
Depending on the size of the battery, every Li-ion battery system has a unique charge-discharge cycle. Quite often, there are three stages in charging; constant current, balance and constant voltage. Overcharging occurs when you try to shove too much current down the anode. Bad charger design can be blamed for this since most battery packs today come with cutoff systems that stop the charging cycle if the batteries heat up.
Batteries Feel Cold, Too
Starting a car in winter requires special efforts, right? Everything has to be thawed before you turn the ignition. This happens in Li-ion batteries, too. They have a narrow temperature range. Consumer-grade batteries, for instance, should not be charged in temperatures below 32 deg.F. Why so? When the temperature of a cell goes down, electrons slow down. This increases the resistance, resulting in a sudden spike in temperature, and, eventually, a fire.
Batteries Age
Over a period of thousands of hours of use, batteries undergo hundreds of thousands of charge-discharge cycles. Each cycle varied, because EVs are, after all, vehicles that navigate a complex environment every day. In traffic, at low speed, battery use is less, but the discharge rate will increase if you’ve got the air conditioner on. Similarly, at high speeds, discharge rates spike, but under heavy braking, charging occurs. Due to this, lithium gets deposited on the anode, much like gunk stuck on a barbeque grill. This, again, will result in a fire.

Road Debris
Most car manufacturers in the U.S. offer something called a ‘skateboard’ design in their EVs. This structure makes use of a floor-mounted battery pack and wheel-mounted motors. The battery management software, regenerative brakes, and cooling systems are placed behind the battery pack. Pretty neat, right? Well, have you ever seen what the underside of a normal car looks like after being driven hard? Road debris, stone chips, pieces of rubber, wood, sand, dirt, pebbles, gravel, snow, wood, and glass are found on every single road in the U.S. If you’re an EV driver, you know where we’re getting at. Yes, damaged separators.
Toxic? Yes!
In school, we were taught that some alkali metals react very strongly if exposed to air. Most lithium-ion batteries also contain cobalt, an alkali. If there is a fire, over a hundred different toxic chemicals are released, which if inhaled, can lead to severe damage. Tests have also revealed that Li-ion battery fires release hydrogen fluoride and phosphoryl fluoride, extremely toxic gases created due to the presence of fluorides in the cell walls, the electrolyte, and the separator.
Let It Burn
If an EV powered by a Li-ion battery catches fire, you don’t have too many options. Most dry fire retardants will not work since the fire is a chain reaction. Firefighters will be exposed to toxic fumes, so they will have to use special breathing equipment. The fire will continue for a period of up to 24 hours if the entire battery pack ignites. A strong, concentrated stream of water is the only solution that’s worked so far. Since this also poses the risk of an electric shock, any firefighting efforts should be carried out in full protective gear. If nothing else works, let the car burn, and stay as far back as possible.
Yes, while EV fire incidents aren’t as common as those seen in gas or diesel-powered cars, the risk is very real. Do realize that since an electric car is completely silent, you won’t be able to hear any tell-tale pops or bangs until it’s too late. Right now, our only hope lies in the development of idiot-proof battery systems that won’t break no matter what.
Sources
https://batteryuniversity.com/learn/archive/lithium_ion_safety_concerns
https://technode.com/2019/06/27/nio-fire-recall-fires/
https://en.wikipedia.org/wiki/Lithium-ion_battery
https://www.economist.com/the-economist-explains/2014/01/27/why-lithium-batteries-keep-catching-fire
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5577247/
https://jalopnik.com/watch-volunteer-firefighters-in-austria-extinguish-a-fi-1819665352
